Analyze the Following Pair of Compounds Which of the Terms
Which of the terms explains the relationship between the two compounds. Compound 2 would have sp3 C-H greater than 3000cm-1.

Lab Reports Samples Google Search Report Template Lab Report Template Lab Report
Diastereomers are stereoisomers that are not related to each other through a reflection operation.

. Enantiomers diastereomers or a OH b HO L R 6. Ch 8 9. Specify which compound in the following pairs of ionic compounds has the higher lattice energy.
The diastereomers include cis - trans E-Z isomers. Select the single best answer. Consider the following pair of compounds.
Up to 256 cash back H- OH H- OH CH20H A 0 B 1 C 2 D 3 E 6 5 Which of the following terms best describes the pair of compounds shown. The left compound has an alcohol hydroxyl and an aldehyde carbonyl group while the right compound has a carboxylic hydroxyl and a carboxylic carbonyl group. Compound 1 would have sp2 C-H less than 3000cm-1.
Compound 2 would have sp3 C-H less than 3000cm-1. Notice how the functional groups are clearly different. Identify the relationship between each of the following pairs.
Start studying Lab 2 Ionic and Covalent Compounds. B LiF or LiBr. Compound 1 would have a smaller fingerprint region because of less hydrogens.
Compound 2 would have peaks indicative of an aromatic system. Solution for Tell how you would analyze the following compounds using IR spectroscopy. Use the following set of terms to describe the relationships between each pair of compounds by placing the correct letters on each line.
OCHCH-CHCOH O CHCHCHCOH Give an acceptable IUPAC name for the following compound. The correct answer is - Diastereomers. Describe the relationship between each pair of compounds.
In options A B and D the pairs of compounds given are not mirror images of each otherSo they are diastereomers. C Peptide dispersed in water. Enantiomers_ diastereomers or the same compound.
Saturday June 5 from 4PM to 5PM PDT. Analyze the following pair of compounds. Molecular compounds contains two elements and ionic compounds contain three of more elements ionic compounds contain.
700 Posted By. Which among the following statements are correct. Question See full Answer.
Be sure to answer all parts. 7 Which of the following terms best describes the pair of compounds shown. Select all that apply.
Analyze the following pair of compounds Which of the terms explains the relationship between the two compounds. The following compounds is the term molecular. Note that IR spectroscopy is VERY MUCH a sporting technique of analysis in that an IR spectrum is open to a lot of interpretation as opposed to non-sporting techniques of analysis such as NMR spectroscopy and X-ray crystallography that can tell you the molecules phone number.
Question 00115712 Subject Chemistry Topic General Chemistry Tutorials. Diastereomers are optically inactive compounds isomers which are not the mirror images of each other. Analyze the following pair of compounds.
AAgNO3 and AgCl bLiOH and Li2SO4 cPbSO4 and BaCO3 dNH4I and Sn3PO42. Follow the structural formulas from left to right to determine that the structures are. Complete step by step solution.
In which of the following pairs of ionic compounds are both members of the pair soluble in water. Chapter 1 Chemistry Homework. They are not mirror images of each other.
Learn vocabulary terms and more with flashcards games and other study tools. Which of the terms explains the relationship between the two. Select all that apply enantiomers epimers anomers diastereomers.
Which of the terms explains the relationship between the two compounds. Sets with similar terms. I both are enantiomers ii both are in threo form iii both are diastereomers.
Each pair of compounds may have more than one relationship or they may have no relationship option F. Alpha α - D - Galactose and Beta β - D - Galactose are examples of cis - trans isomers also known as diastereomers. For each of the following pairs of compounds determine which compound is more stable you may find it helpful to draw out the chair conformations.
Analyze the types of potential energy being used by an athlete competing in each of these athletic events. The answer is noand OH absorptions are very prominent in the IR spectrum. 10102015 0933 AM Due on.
COH Identify the more acidic compound in the following pair. CH3 H C OH 3. Analyze the following pair of compounds.
For each of the following pairs of. When iron II sulphate is reacted with the ammonium hydroxide then it gives green colour precipitates and on the reaction with iron III sulphate it gives orange precipitates due to the formation of two and three hydroxide ions respectively in the reaction which is clearly visible through the difference in the. Select all that apply Select.
Terms may be used more than once or not at all.

Investigation On Conductors And Insulators 1 Lesson Plan Go To Http Www Saveteacherssundays Com Scienc Teaching Resources Primary Lesson Plants Lesson Plans
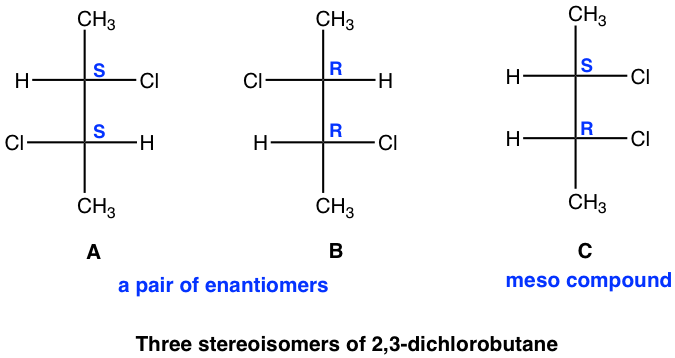
5 6 Compounds With More Than One Chirality Centers Organic Chemistry I

Chemistry Articles For Use With Google Slides Distance Learning Articles Activities Distance Learning Teacher Resources

Chemical Bonding Molecular Shapes And Vsepr Theory Britannica

Create A Concept Map Of Biomolecules Concept Map Biology Activity Graphic Organizers

Covalent Bond Definition Properties Examples Facts Britannica

Coordinate Covalent Bond Definition Examples Formation And Properties Covalent Bonding Coordinates Bond

Physical Chemical Properties Physical And Chemical Properties Collaborative Learning Physics

Urinary System Activity Human Body Systems Cootie Catcher Review Game In 2022 Urinary System Activities Human Body Systems Body Systems

Coordination Compound Ligand Field And Molecular Orbital Theories Britannica

Anorganische Chemie Anorganische Chemie Organic Chemistry Study Organic Chemistry Chemistry Education

Vsepr Chart Valence Shell Electron Pair Repulsion Theory

Endothermic And Exothermic Worksheets And Activities With Answers Exothermic Reaction How To Memorize Things Chemistry Lessons

Chemistry Articles For Use With Google Slides Distance Learning Articles Activities Distance Learning Teacher Resources
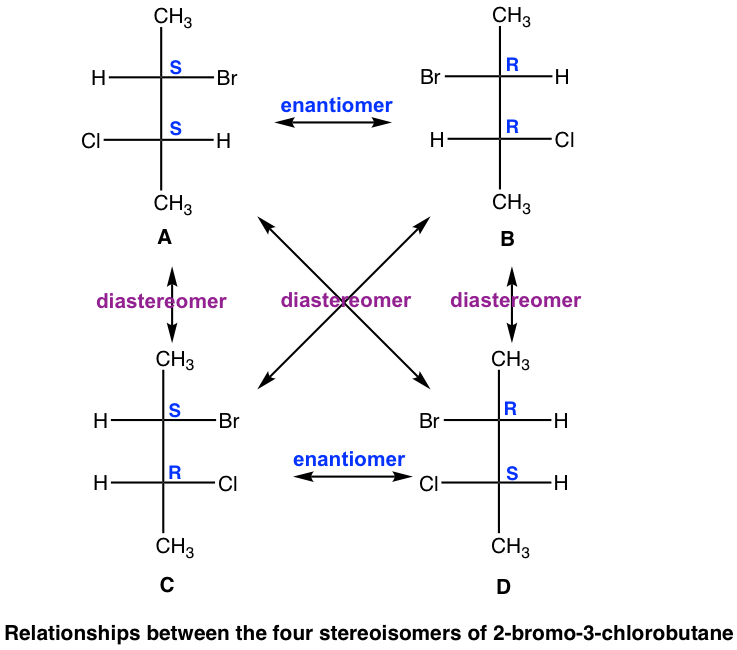
5 6 Compounds With More Than One Chirality Centers Organic Chemistry I

Gcse Edexcel Chemistry Key Concepts In Chemistry Covalent Bonding Complete Revision Summary Notes Vi Covalent Bonding Gcse Chemistry Chemistry

Boom Cards Thermal Energy Vocabulary 3 Ways To Review Thermal Energy Vocabulary Energy Transfer

Graphical Abstract Mean Free Path Thermoelectric Materials Engineering

Comments
Post a Comment